With the increase in the aging population, an effective therapeutic regimen is still an unmet medical need for different neurodegenerative disorders. Dr. Joseph Jen-Tse Huang, an associate research fellow at the Institute of Chemistry in Academia Sinica, Dr. Pang-Hsien Tu, a former assistant research fellow at the Institute of Biomedical Science, and their research teams recently report a self-assembled amphiphilic peptide that can inhibit protein aggregation implicated in the pathogenesis of Huntington’s disease (HD). This new finding was published in Advanced Science on January, 2020.
Current literature indicates the oligomerization and aggregation of the mutant Huntingtin (mHtt) protein are closely related to HD proteinopathy. However, for the difficulties in observing the dynamic aggregation and oligomerization process of mHtt in vivo, the evaluation of potential drugs is usually restricted. By combing lifetime-based fluorescence microscopies and biophysical tools, Dr. Huang and Dr. Tu demonstrate the designed amphiphilic peptide, which targets the mHtt at an early stage, can perturb the oligomer assembly process nanoscopically, suppress the amyloid property of mHtt, and ameliorate mHtt-induced neurological damage in cell and mouse models.
Dr. Huang remarked, “While tracking the transient and subtle changes in protein conformational dynamics in vitro is feasible, observing the aforementioned phenomenon in live cells remains challenging. By establishing a combined biophotonic platform, we are the first to visualize not only the aggregation but also the oligomerization process perturbed by the amphiphilic peptide in live neuronal cells in real time.” Moreover, they found the amphiphilic peptide is able to transport to the brain and rescue the memory deficit through intranasal administration. The related patent in this cooperative and multidiscipline study has just been approved in Taiwan and under application in other country. This study provides new insight into the design of a targeted therapeutic agent and creates a biophotonic platform for monitoring the oligomerization process in neurodegenerative diseases.
Dr. Ruei‐Yu He is the first author in this study. The corresponding authors include Dr. Joseph Jen-Tse Huang, Dr. Ruei‐Yu He, and Dr. Pang-Hsien Tu. Dr. Huang also appreciates the contributions from Dr. Jen-Kun Chen and Dr. Yung-Feng Liao from the Institute of Biomedical Engineering and Nanomedicine in National Health Research Institutes and the Institute of Cellular and Organismic Biology in Academia Sinica.
The full article entitled “Nanoscopic Insights of Amphiphilic Peptide against the Oligomer Assembly Process to Treat Huntington’s Disease” can be now found at the Advanced Science website at: https://onlinelibrary.wiley.com/doi/full/10.1002/advs.201901165
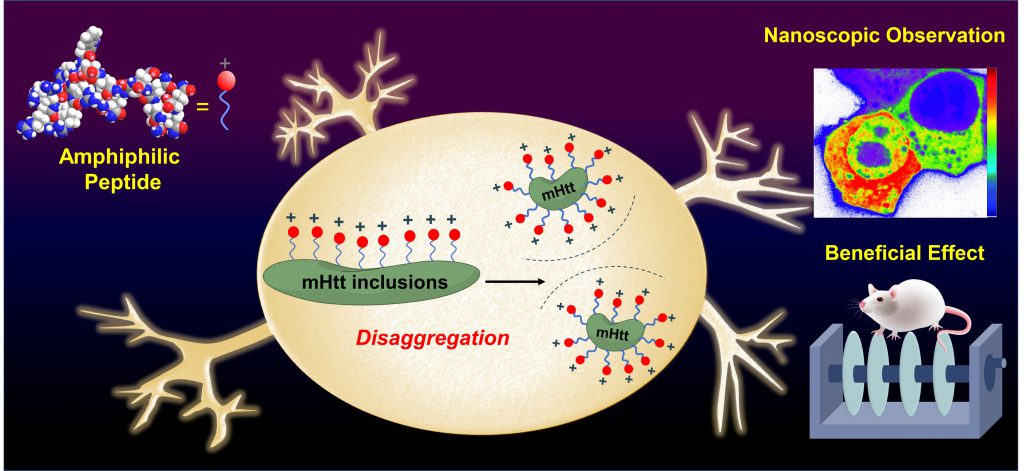
Figure 1. The amphiphilic peptide can perturb the oligomer assembly of mHtt through the charge repulsion, ameliorate mHtt-induced neurological damage, and rescue the memory deficit.
