A new discovery has unearthed a group of small regulatory RNAs, known as the mir-17~92 cluster, that protects motor neurons, a cell type that is particularly sensitive to amyotrophic lateral sclerosis (ALS). This discovery could provide new opportunities for designing therapies for this disease. The study “Mir-17~92 Confers Differential Vulnerability of Motor Neuron Subtypes to ALS-associated Degeneration” has been published in the journal Cell Stem Cell.
ALS is a neurodegenerative disorder that progresses rapidly. The disease attacks the motor neurons controlling voluntary muscle movement. Intriguingly, not all motor neurons degenerate to the same degree. Approximately 70% of ALS patients experience fast degeneration of the motor neurons controlling their leg and arm muscles. These patients lose the ability to stand and walk, and to complete everyday tasks. Ultimately, patients die from breathing difficulties due to degeneration of the motor neurons controlling the diaphragm. Unfortunately, there is no effective cure for this devastating disease.
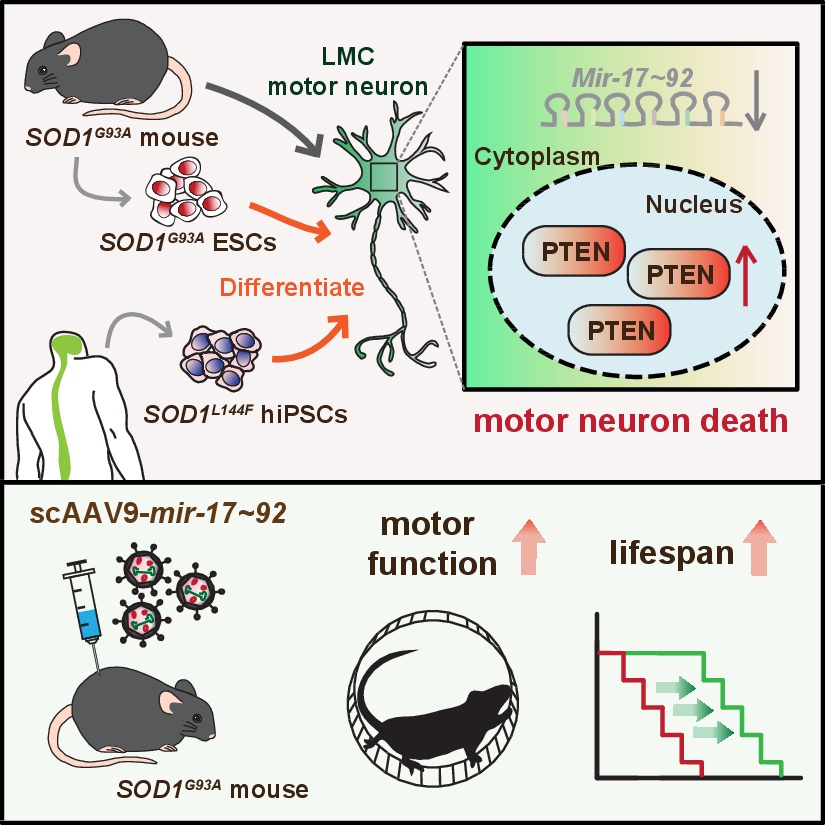
The research team led by Dr. Jun-An Chen PhD at the Institute of Molecular Biology, Academia Sinica (Taiwan), focuses on a short form of RNA, namely microRNAs (miRNAs). The team has previously found that the mir-17~92 cluster, a group of six miRNAs, is more abundant in the limb-controlling motor neurons of developing embryos. These miRNAs cooperate to prevent the accumulation of PTEN proteins in the nucleus, thereby protecting neurons from cell death and ensuring that sufficient limb-controlling motor neurons develop during embryogenesis.

In this new study, the team revealed a critical role for mir-17~92 in adults, and unveiled its role in regulating motor neuron degeneration during ALS disease. The authors used several cell and mouse models, including a classical ALS SOD1G93A mouse model, embryonic stem cell-derived motor neurons from SOD1G93A mice, as well as human ALS SOD1L144F motor neurons derived from induced pluripotent stem cells. Results from all these models consistently show that reduced mir-17~92 levels, together with accumulation of detrimental nuclear PTEN protein, occur prior to the death of ALS limb-controlling motor neurons. The team was able to restore mir-17~92 levels in human and mouse ALS motor neurons by a genetic approach or via virus-mediated gene therapy. Excitingly, abnormal nuclear PTEN levels were reversed and motor neuron numbers recovered to normal levels using these approaches. Furthermore, motor deficits and lifespans of adult SOD1G93A mice were remarkably improved after single treatment with virus-delivered mir-17~92.
In addition to studying ALS linked to SOD1 mutation, Dr. Chen’s laboratory is continuing to test the rescue effects of mir-17~92 in motor neurons associated with other genetic or non-genetic causing types of human ALS disease. Dr. Chen noted “Given that global miRNA dysregulation has been observed in motor neurons from different types of ALS patients, we are optimistic that mir-17~92 gene therapy can also be applied to diverse ALS types.”
The study is funded by Academia Sinica, National Health Research Institutes and the Ministry of Science and Technology in Taiwan.