Newly discovered protein quality control mechanism to create new opportunities in bioengineering
Many human diseases such as aging, degenerative neurological diseases, and cancer are related to the defects in proteins. To remove defective proteins, cells prune themselves through a quality control mechanism that separates the wheat from the chaff.
A group led by Dr. Hsueh-Chi Sherry Yen, Associate Research Fellow at the Institute of Molecular Biology of Academia Sinica, has uncovered an important quality control degradation system in human cells—a protein complex known as CRL2 that specifically recognizes, marks, and destroys aberrant proteins bearing unusual terminal sequences. The discovery of this quality control mechanism has set a milestone in the study of protein degradation control, offering new ideas that spur further innovation in the field protein bioengineering while also paving the way for new medical treatments for illnesses associated with protein quality defects. Their findings were published in Molecular Cell in May 2018 and also selected as a featured article of this issue.
Proteins are the basic building blocks; they enable cells to perform physiological functions necessary for life. Human cells are made up of tens of thousands of proteins. However, these proteins are also susceptible to defects in the replication process or due to environmental stimuli. Defective proteins may affect the function of other normal proteins, thereby harming cells and even the entire organism. Maintaining the healthy function of proteins is thus of utmost importance for cell survival.
Protein quality control degradation systems rid the cell of aberrant proteins, preventing detrimental effects on normal cellular function. This quality control function degrades the defective protein into harmless peptides or amino acids. However, only a few proteins in the human body have quality control functions. How do these special quality control proteins accurately identify the distinct patterns of the numerous types of defective proteins and ferret them out? This is the main focus area of Dr. Hsueh-Chi Sherry Yen and her research team.
In 2008, Dr. Hsueh-Chi Sherry Yen established the Global Protein Stability (GPS) monitoring system, which can simultaneously detect the stability of tens of thousands of proteins located in cells. In 2015, Dr. Yen and her research team first discovered that among selenoproteins, the CRL2 protein has special quality control functions. In this recent study, her research team used this Global Protein Stability technology to further understand how the quality control mechanism in CRL2 operates.
Their study found that by taking advantage of various interchangeable substrate receptors, CRL2 can quickly and accurately identify specific sequences of short peptides at the end of defective proteins, greatly expanding the range of aberrant proteins recognized by CRL2. Upon recognition, CRL2 then marks and removes these defective proteins. These short peptide sequences that CRL2 recognizes consist of about 6 to 10 amino acids (as shown in the figure below). Different sequences of short peptides also prime different rates of degradation. This newest research study pinpointed with further accuracy the degradation rates of regulating proteins, and it is expected to bring new opportunities and applications in protein bioengineering.
Besides targeting defective proteins, Dr. Yen and her research team also found that the quality control mechanism of CRL2 can also be applied to proteins in normal cells with natural occurring degradation sequences. As such, any protein that is attached to these short peptides can be quickly and accurately detected, identified, marked, and then degraded by CRL2. At present, the research team has begun to examine other physiological functions of this degradation mechanism as well as its overall impact on the protein.
The leading author of this paper published in Molecular Cell is Hsiu-Chuan Lin, a doctoral student in the Genome and Systems Biology (GSB) Degree Program at the National Taiwan University. This research project conducted by Dr. Yen’s lab is supported by funding provided by the Career Development Award from Academia Sinica and grants from the Ministry of Science and Technology in Taiwan.
For more details on this study, the full research article entitled “C-Terminal End-Directed Protein Elimination by CRL2 Ubiquitin Ligases” is available at https://www.cell.com/molecular-cell/abstract/S1097-2765(18)30276-4.
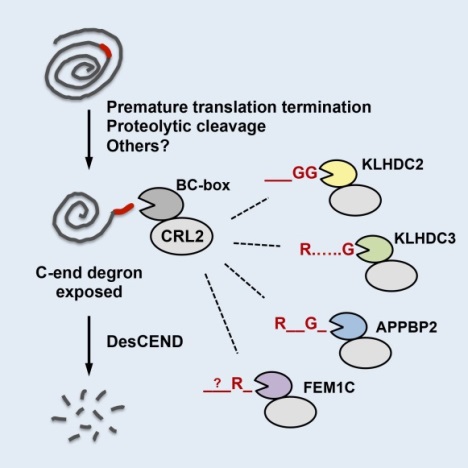
The above is a schematic representation of CRL2 involved in the protein degradation mechanism and the characteristics of the specific short peptides recognized by CRL2.
