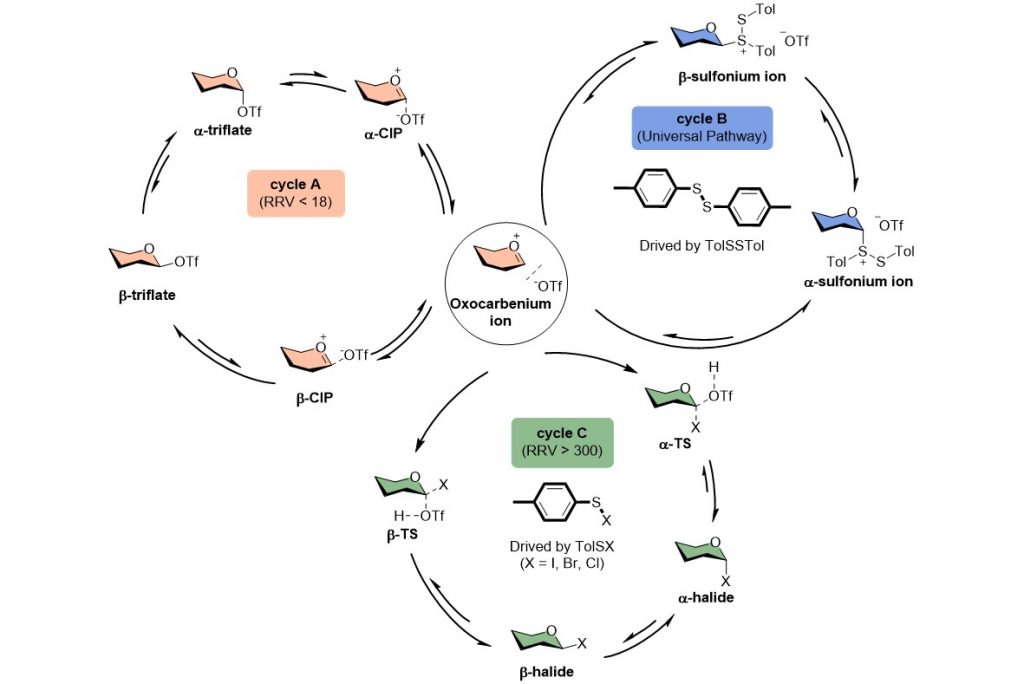
Chemical synthesis is one of the most reliable methods to access high-purity homogeneous oligosaccharides and their derivatives in large quantities. However, achieving high stereoselectivity in glycosidic bond formation remains a significant challenge in carbohydrate synthesis. This challenge arises from a limited understanding of the reaction mechanism with numerous unknown effects, which is an area that has not been thoroughly explored and clarified. A research team led by Dr. Cheng-Chung Wang at the Institute of Chemisty, Academia Sinica, conducted an extensive investigation into the glycosylation mechanism, employing low-temperature Nuclear Magnetic Resonance (NMR) and statistical methodologies. Our research led to the discovery of a novel pathway driven by counterion exchanges and reaction byproducts, which elucidates the contributions of intermediates to stereochemistry. Additionally, we introduced relative reactivity values (RRVs), acceptor nucleophilic constants (Aka), and Hammett substituent constants (σ values) as comprehensive indicators of mechanistic pathways. These findings have the potential to significantly simplify the optimization of building blocks and reaction conditions in carbohydrate synthesis. This study has been published on October 18, 2023 in Science Advances.
Article link: https://www.science.org/doi/10.1126/sciadv.adk0531
Related link: https://www.chem.sinica.edu.tw/research.php?menuT=wangcc_res_2023_2&lang=en